The European Health Data Space (EHDS), established under Regulation (EU) 2025/327, is a framework that defines rules, common standards and practices, data-sharing infrastructure, and governance with an explicit goal to facilitate pan-European access to medical data for primary and secondary uses and the creation of a European Single Market for digital health systems. Its implementation is set to unfold over the coming years, with key milestones expected between 2025 and 2030 to ensure a progressive and coordinated rollout across Member States.
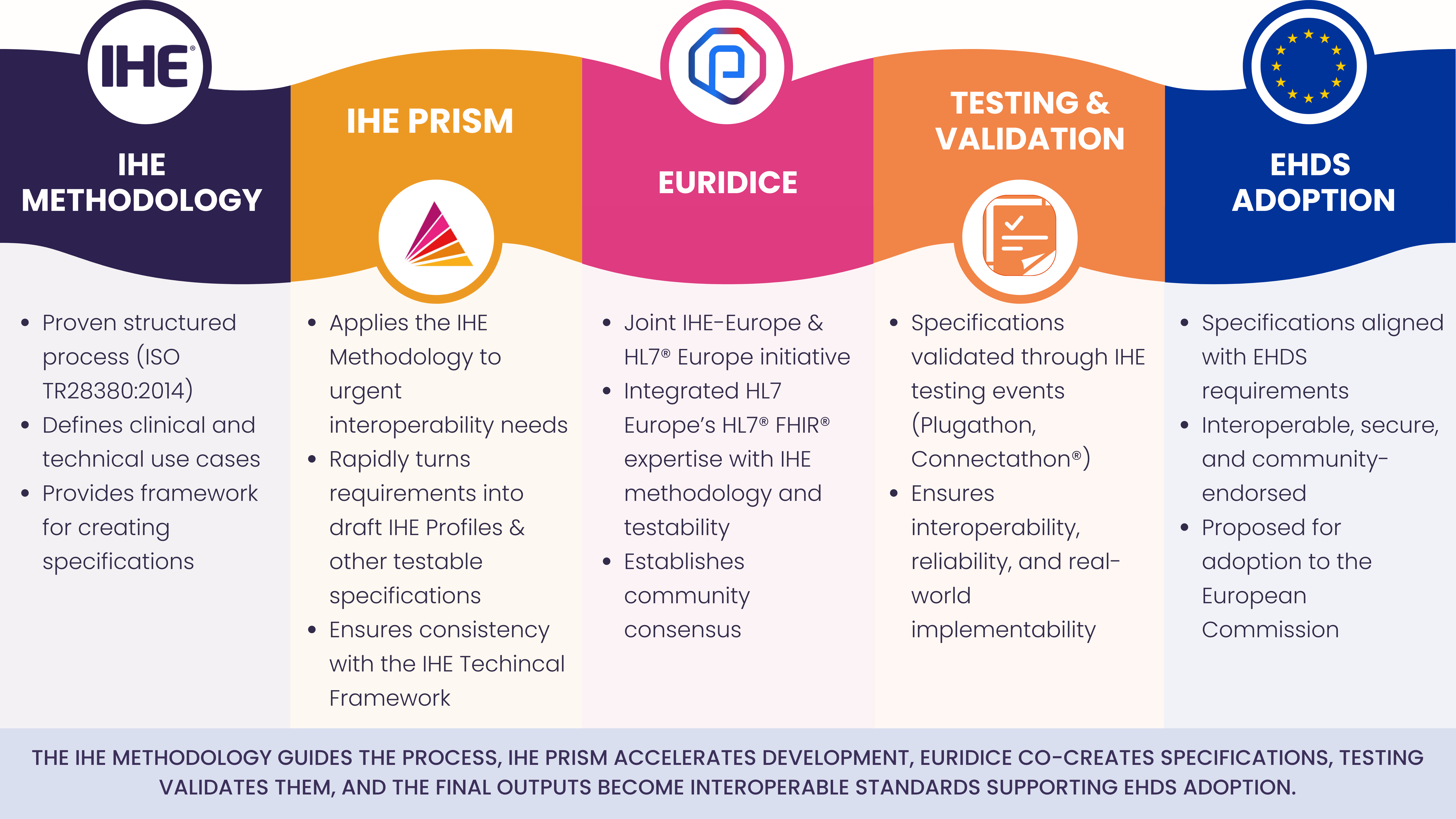
IHE can play a pivotal role by leveraging the IHE™ Methodology (ISO TR28380:2014), successfully adopted by many organizations around the world. IHE-Euorope has also participated in the development of use cases and specifications for the EEHRxF, including European Commission projects such as XeHealth, UNICOM, and XpanDH.
More recently, IHE-Europe has implemented concrete steps and initiatives to facilitate the implementation of the EHDS, more specifically:
IHE PRISM EHDS
IHE-Europe has established an IHE PRISM focusing on the European Health Data Space (EHDS), where fast, coordinated specification development is needed to support pan-European data sharing. By leveraging existing IHE Profiles, exploring new specifications where required, and aligning outputs with the IHE testing continuum, the initiative provides implementable, verifiable specifications for healthcare providers, vendors, and policymakers. Collaborating with partner SDOs such as HL7® Europe, IHE PRISM accelerates the creation of interoperable, secure, and scalable solutions to support the EHDS rollout between 2025 and 2030.
EURIDICE
The EURIDICE initiative, a joint effort between IHE-Europe and HL7 Europe,. EURIDICE creates well-architected, implementable specifications that can be leveraged by the European Commission for reference in the EHDS Implementing Acts. By combining HL7 Europe’s HL7® FHIR® expertise with IHE’s methodology and testing approach, EURIDICE turns ideas into strong, community-validated artifacts, supporting implementation, validation, and alignment across EU Member States, EU-funded projects, and SDO processes.
In support of EURIDICE, IHE Europe is rolling out an IHE PRISM, leveraging its innovative mechanics to accelerate IHE Technical Framework coverage of EHDS requirements.
- The IHE Methodology provides a proven structured process and framework for specifications.
- EHDS IHE PRISM provides the mechanics to rapidly develop additional IHE Profiles and supplementary testable specifications in support of EHDS adoption .
- EURIDICE leverages this process, delivering community-endorsed and through testing events well validated specifications;
- Specifications created through the EURIDICE initiative are proposed for adoption for the European Health Data Space to the European Commission.
IHE-Europe White paper
Due to the intricate challenges of achieving interoperability across diverse health systems and infrastructures, IHE-Europe is thrilled to present its latest whitepaper, "Leveraging the IHE Methodology to Accelerate the Successful Implementation of the European Health Data Space (EHDS)." This document showcases how IHE’s proven methodology, including IHE Profiles and conformance testing frameworks, supports seamless, secure, and scalable health data sharing across Europe. By addressing interoperability challenges and building on existing infrastructure, the white paper offers a clear path to achieving the ambitious EHDS vision.
The EHDS is a fast-evolving ecosystem. To keep up with these developments, this page will be updated regularly. Be sure to check back frequently to stay informed on IHE-Europe updates.




